Clinical
EVIDENCE
IDE Trial Enrollment Completed
In October 2025, Emboline completed enrollment in the Protect the Head to Head IDE trial (NCT05684146), which is a 500 patient, prospective, randomized, open label, multicenter, 2-arm study to demonstrate safety and effectiveness of the Emboliner® Embolic Protection Catheter compared to a control device (SentinelTM CPS, Boston Scientific) for patients undergoing TAVR. Data from the IDE study will be used to file for FDA commercial approval in the U.S. and CE mark in Europe. Trial results will be presented at an upcoming conference.
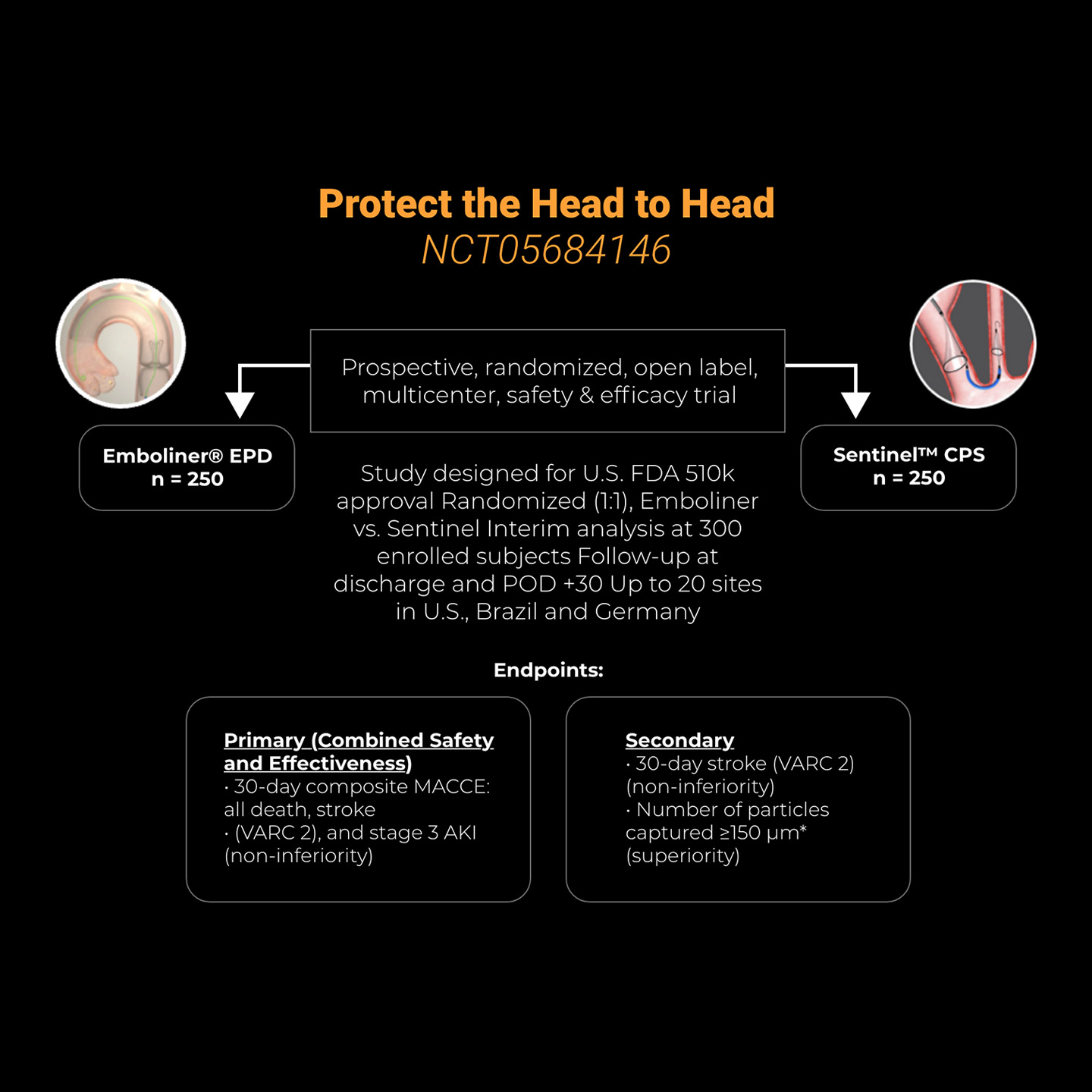
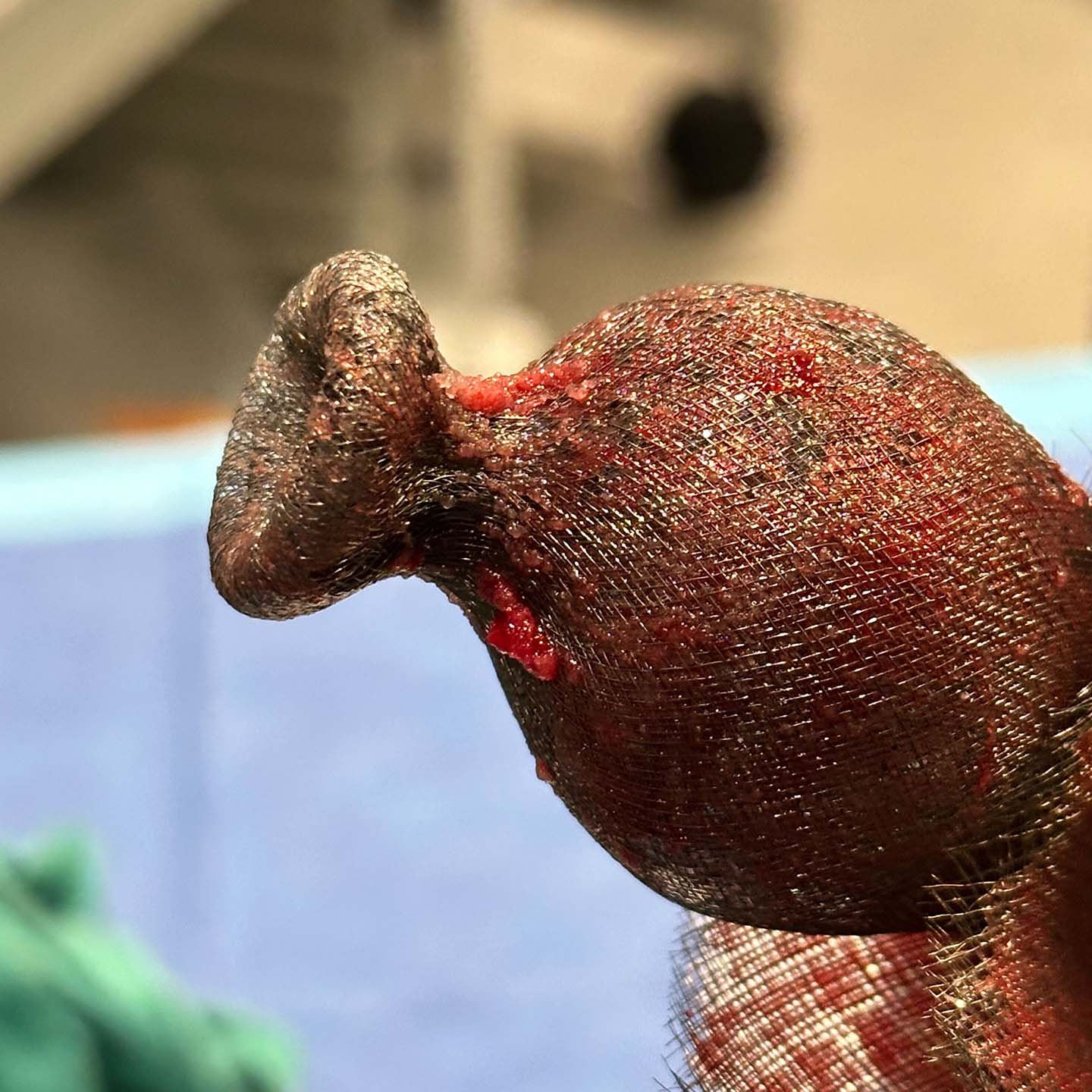
Early Feasibility Studies Demonstrated Safety and Efficacy
In the SafePass series of early feasibility studies (EFS), the Emboliner has been demonstrated to be safe, effective and easy to use, with superior debris capture when compared to historical data. 1
References:
- 1 Grubman D, et al. Predictors of Cerebral Embolic Debris During Transcatheter Aortic Valve Replacement: The SafePass 2 First-in-Human Study. Am J Cardiol. 2023 Nov 15;207:28-34.
